Lorenzo Romano Amedeo Carlo Avogadro (August 9, 1776–July 9, 1856) was an Italian savant chemist, most noted for his contributions to the theory of molarity and molecular weight. During his stay in Vercelli, he wrote a concise note (memoria) in which he declared the hypothesis of. Amedeo Avogadro Lornezo Romano Amedeo Carlo Avogadro di Quaregna e di Cerretowas a famous creation scientist best known for his contributions to chemistry. He was born in Turin, and his family was also well-known as a lawyer in Italy. He fame is derived from his contribution about molecular law, known as Avogadro's lawand Avogadro's number. Lived 1776 – 1856. Amedeo Avogadro is best known for his hypothesis that equal volumes of different gases contain an equal number of molecules, provided they are at the same temperature and pressure. His hypothesis was rejected by other scientists. It only gained acceptance after his death. More contributions of Amadeo Avogadro to quantitative aspect of chemistry. Amedeo Avogadro is best known for his hypothesis that equal volumes of different gases contain an equal number of mo view the full answer. Previous question Next question. The paper’s author was Italian mathematical physicist Amedeo Avogadro, and his idea became known as Avogadro’s law, which is now a fundamental concept in the physical sciences. Avogadro’s idea, however, was not initially accepted. Sing karaoke by smule for mac.
ItalianLorenzo Romano Amedeo Carlo Avogadro, Count of Quaregna and Cerreto (9 August 1776, Turin, Piedmont-Sardinia – 9 July 1856), was an Italian scientist.

His most noted for his contribution to molecular theory now known as Avogadro's law, which states that equal volumes of gases under the same conditions of temperature and pressure will contain equal numbers of molecules. The matrix screensaver for mac os x.
Avogadro was born at Turin to a noble family of Piedmont-Sardinia in the year 1776. That city, now part of Italy, was then of the Kingdom of Sardinia.
He graduated in ecclesiastical law at the late age of 20 and began to practice. Soon after, he dedicated himself to physics and mathematics.
In 1820, he became a professor of physics at the University of Turin.
Little is known about Avogadro's private life, which appears to have been sober and religious. He married Felicita Mazzé and had eight children.
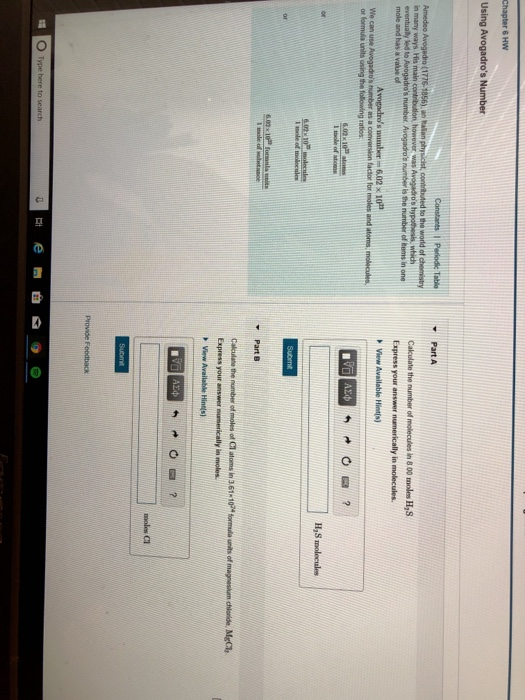
In honor of Avogadro's contributions to molecular theory, the number of molecules in one mole was named Avogadro's number, NA or 'Avogadro's constant'. It is approximately 6.0221415×1023.
Amedeo Avogadro Contribution To Chemistry
Avogadro's number is used to compute the results of chemical reactions. It allows chemists to determine amounts of substances produced in a given reaction to a great degree of accuracy.
Avogadro's Law states that the relationship between the masses of the same volume of same gases (at the same temperature and pressure) corresponds to the relationship between their respective molecular weights.
One of his most important contributions was clearly distinguishing one from the other, stating that gases are composed of molecules, and these molecules are composed of atoms.
Avogadro is hailed as a founder of the atomic-molecular theory.
Only through studies by Charles Frederic Gerhardt and Auguste Laurent on organic chemistry was it possible to demonstrate that Avogadro's law explained why the same quantities of molecules in a gas have the same volume.

Johann Josef Loschmidt first calculated the value of Avogadro's number, often referred to as the Loschmidt number in German-speaking countries (Loschmidt constant now has another meaning).
Amedeo Avogadro Contribution To Chemistry
In 1911, a meeting in Turin commemorated the hundredth anniversary of the publication of Avogadro's classic 1811 paper. King Victor Emmanuel III attended. Thus, Avogadro's great contribution to chemistry was recognized.
Rudolf Clausius, with his kinetic theory on gases proposed in 1857, provided further evidence for Avogadro's Law. Jacobus Henricus van 't Hoff showed that Avogadro's theory also held in dilute solutions.
He died on 9 July 1856.
Source: Link
In 1811 Avogadro put forward a hypothesis that was neglected by his contemporaries for years. Eventually proven correct, this hypothesis became known as Avogadro’s law, a fundamental law of gases.
The contributions of the Italian chemist Amedeo Avogadro (1776–1856) relate to the work of two of his contemporaries, Joseph Louis Gay-Lussac and John Dalton. Gay-Lussac’s law of combining volumes (1808) stated that when two gases react, the volumes of the reactants and products—if gases—are in whole number ratios. This law tended to support Dalton’s atomic theory, but Dalton rejected Gay-Lussac’s work. Avogadro, however, saw it as the key to a better understanding of molecular constituency. Downloading paint for mac.

Avogadro’s Hypothesis
In 1811 Avogadro hypothesized that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. From this hypothesis it followed that relative molecular weights of any two gases are the same as the ratio of the densities of the two gases under the same conditions of temperature and pressure. Avogadro also astutely reasoned that simple gases were not formed of solitary atoms but were instead compound molecules of two or more atoms. (Avogadro did not actually use the word atom; at the time the words atom and molecule were used almost interchangeably. He talked about three kinds of “molecules,” including an “elementary molecule”—what we would call an atom.) Thus Avogadro was able to overcome the difficulty that Dalton and others had encountered when Gay-Lussac reported that above 100°C the volume of water vapor was twice the volume of the oxygen used to form it. According to Avogadro, the molecule of oxygen had split into two atoms in the course of forming water vapor.
bio-avogadro.jpg
Edgar Fahs Smith Collection, Kislak Center for Special Collections, Rare Books and Manuscripts, University of Pennsylvania
Curiously, Avogadro’s hypothesis was neglected for half a century after it was first published. Many reasons for this neglect have been cited, including some theoretical problems, such as Jöns Jakob Berzelius’s “dualism,” which asserted that compounds are held together by the attraction of positive and negative electrical charges, making it inconceivable that a molecule composed of two electrically similar atoms—as in oxygen—could exist. In addition, Avogadro was not part of an active community of chemists: the Italy of his day was far from the centers of chemistry in France, Germany, England, and Sweden, where Berzelius was based.
Personal Life
Avogadro was a native of Turin, where his father, Count Filippo Avogadro, was a lawyer and government leader in the Piedmont (Italy was then still divided into independent countries). Avogadro succeeded to his father’s title, earned degrees in law, and began to practice as an ecclesiastical lawyer. After obtaining his formal degrees, he took private lessons in mathematics and sciences, including chemistry. For much of his career as a chemist he held the chair of physical chemistry at the University of Turin.
The information contained in this biography was last updated on November 30, 2017.
